Vascular Surgery Research
Vascular Surgery Research
Publications
Our faculty all engage in research - in 2023 we published a total of 91 manuscripts. Visit our Research Investigator Profiles to learn more.
Clinical Trials

Nectero Endovascular Aneurysm Stabilization Treatment
- PI: Benjamin Starnes
- Description: A clinical study investigating a new treatment for patients with small to medium size aneurysms (3.5-5.0 cm in diameter). The treatment involves a one-time minimally invasive procedure where a drug is delivered to the aneurysm wall. The potential benefits include slowing aneurysm growth rate and lowering the risk of rupture
Boomerang Trial
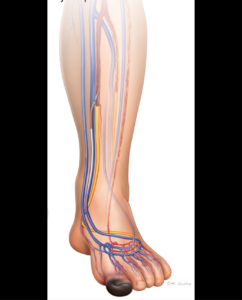
- PI: Kirsten Dansey
- Description: A trial investigating a minimally invasive method for increasing blood flow to the lower leg. The goal is to prevent lower leg amputation and promote wound healing. This study includes patients with chronic limb threatening ischemia where they have severe foot pain at rest or non-healing foot wounds.
PMEG: Physician Modified Endovascular Grafts
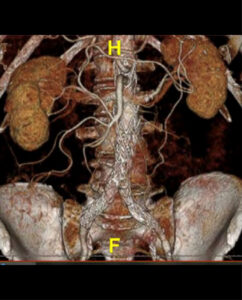
- PI: Benjamin Starnes
- Description: A minimally invasive operation to repair juxtarenal aortic aneurysms that is proven safe and effective for patients unsuitable for open surgical repair. Due to the unique nature of the procedure, Dr. Starnes had to obtain an Investigational Device Exemption (IDE) from the FDA to be able to do these procedures and track subsequent outcomes. This was the first IDE of its kind in the United States and Dr. Starnes has enrolled over 200 patients in the trial to date.
ViTAA
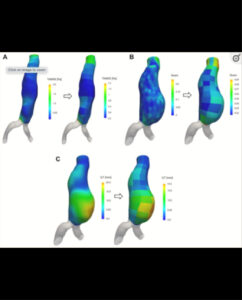
- PI: Benjamin Starnes
- Description: This is a Prospective Registry study to collect imaging and clinical data both on patients with aortic aneurysm disease undergoing serial monitoring and on patients pre and post-endovascular repair, using ViTAA (The Sponsor) aortic mapping technology.
ELEGANCE: Drug-Eluting REGistry: Real-World Treatment of Lesions in the Peripheral Vasculature
- PI: Elina Quiroga
- Description: The ELEGANCE Registry's objective is to collect Real-World Data (RWD), including populations previously not represented in Peripheral Vascular Disease (PVD) trials, health economics data, and to support the safe use of commercially available Boston Scientific Corporation (BSC) drug-eluting devices for the treatment of lesions located in the peripheral vasculature.
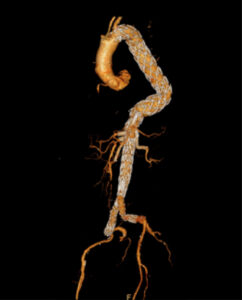
BTEVAR: Branched Thoracic Endovascular Grafts for the Treatment of Thoraco-abdominal Aortic Aneurysms
- PI: Matthew Sweet
- Description: An investigator-initiated, prospective, consecutively enrolling, non-randomized single institution clinical evaluation of the safety and effectiveness of branched and fenestrated-branched endovascular stent grafts to preserve branch vessels when used in the treatment of patients with thoraco-abdominal aortic aneurysms. The study evaluates non-FDA-approved off the shelf and custom made branched and fenestrated-branched stent grafts manufactured by Cook Medical.
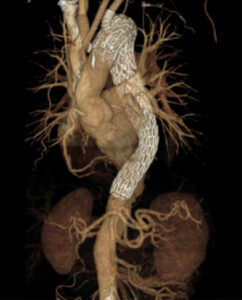
GORE TBE: Thoracic Branch Endoprosthesis
- PI: Matthew Sweet
- Description: The objective of this study is to determine whether the GORE® TAG® Thoracic Branch Endoprosthesis is safe and effective in treating lesions of the aortic arch and descending thoracic aorta, requiring Zone 2 proximal implantation of the device.

GORE TAMBE: Thoracoabdominal endoprosthesis
- PI: Matthew Sweet
- Description: The purpose of this study is assess the safety and effectiveness of the GORE® EXCLUDER® Thoracoabdominal Branch Endoprosthesis (TAMBE) Device in the treatment of thoracoabdominal and pararenal aortic aneurysms.

Bolton Relay Pro
- PI: Niten Singh
- Description: This clinical trial is a prospective, multicenter, non-blinded, non-randomized study designed to assess the RelayPro thoracic endografts in the treatment of acute, complicated type B aortic dissection. The primary endpoint will measure all-cause mortality at 30 days post-procedure.
Multi-disciplinary Thoracic Aortic Program
Diseases of the thoracic aorta are life-changing diagnoses. The management of these conditions is complex and it is normal for patients and their families to feel overwhelmed by the prospect of major surgery as well as to worry about the long-term implications of this disease. We are here to help.
At the University of Washington “MTAP,” we believe that complex thoracic aortic disease is best treated by a team-based approach. We understand that no individual provider can tackle these complex issues alone, but rather acknowledge the benefit of a multi-disciplinary approach utilizing the diverse specialists available at the University of Washington. Our team includes both cardiac and vascular surgeons, along with collaborators in cardiology and medical genetics.
