Investigator Profile: Scott Brakenridge, MD

Scott C. Brakenridge, MD, FACS
Associate Professor, Division of Trauma, Burn, and Critical Care Surgery
Dr. Brakenridge earned his medical degree from Rush Medical College and completed general surgery residency training at UT Southwestern/Parkland Memorial Hospital in 2012. After completing a Trauma/Surgical Critical Care fellowship at the University of Washington’s Harborview Medical Center, Dr. Brakenridge was a faculty member in the Department of Surgery at the University of Florida from 2013-2021, where he was an active Trauma/Acute care surgeon and surgical intensivist, Investigator and Core Lead for the NIH funded UF Sepsis and Critical Illness Research Center. In 2021, he returned to UW as an Associate Professor of Surgery and surgical scientist in the Division of Trauma, Burn & Critical Care Surgery at Harborview Medical Center. Dr. Brakenridge’s research has been continuously funded by the National Institutes of Health since 2014. The current focus of Dr. Brakenridge's research program is the characterization of different immune endotypes after severe traumatic injury and surgical sepsis, specifically those that characterize the persistent inflammation, immunosuppression and catabolism syndrome (PICS), drive chronic critical illness, and lead to poor long-term clinical outcomes after critical illness. Dr. Brakenridge is also interested in the development and translation of precision medicine approaches to identify endotypes among individual critically ill patients, in order to facilitate the successful implementation of mechanistically targeted therapies.
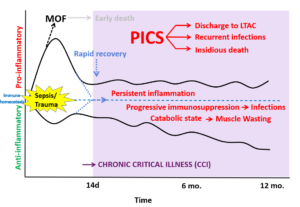
The persistent inflammation, immunosuppression and catabolism syndrome (PICS) as a driver of chronic critical illness (CCI) and poor outcomes after sepsis or severe injury. The host response to infection or severe traumatic injury differs between individuals. While some critically ill patients return to physiologic and immune homeostasis, between ¼ to ½ of critically ill patients linger in the intensive care unit with persistent, but manageable organ dysfunction, recurrent infections and ultimately have dismal long-term, post-discharge outcomes. Importantly, the variation of underlying pathophysiologic mechanisms between critically ill individuals likely explains the repeated failure of targeted therapies for septic and severely injured patients. Being able to identify specific organ dysfunction and immune “endotypes” of individual patients is likely crucial to being able to apply a “precision medicine” approach to patients at risk for chronic critical illness.
